Thermochemistry Heat Of Reaction Lab Report . this experiment is an introduction to the basic principles of thermochemistry and involves the. The purpose of this lab is to use calorimetry to measure the heats of reaction, while also attempting to use the. the heat produced can be used to determine the molar heat of neutralization (δhneut) resulting in the heat released per mole of. Ca (s) + hcl (aq) following the procedure of the sample calculation in part 2, calculate the molar heat of. thermochemistry is the study of how much energy is absorbed or produced by a chemical reaction. the amount of heat that the system gives up to its surroundings so that it can return to its initial temperature is the. The sources of heat exchanged by the. use the quantities described below to calculate the heat of each reaction.
from www.slideshare.net
The purpose of this lab is to use calorimetry to measure the heats of reaction, while also attempting to use the. thermochemistry is the study of how much energy is absorbed or produced by a chemical reaction. use the quantities described below to calculate the heat of each reaction. Ca (s) + hcl (aq) following the procedure of the sample calculation in part 2, calculate the molar heat of. this experiment is an introduction to the basic principles of thermochemistry and involves the. the amount of heat that the system gives up to its surroundings so that it can return to its initial temperature is the. The sources of heat exchanged by the. the heat produced can be used to determine the molar heat of neutralization (δhneut) resulting in the heat released per mole of.
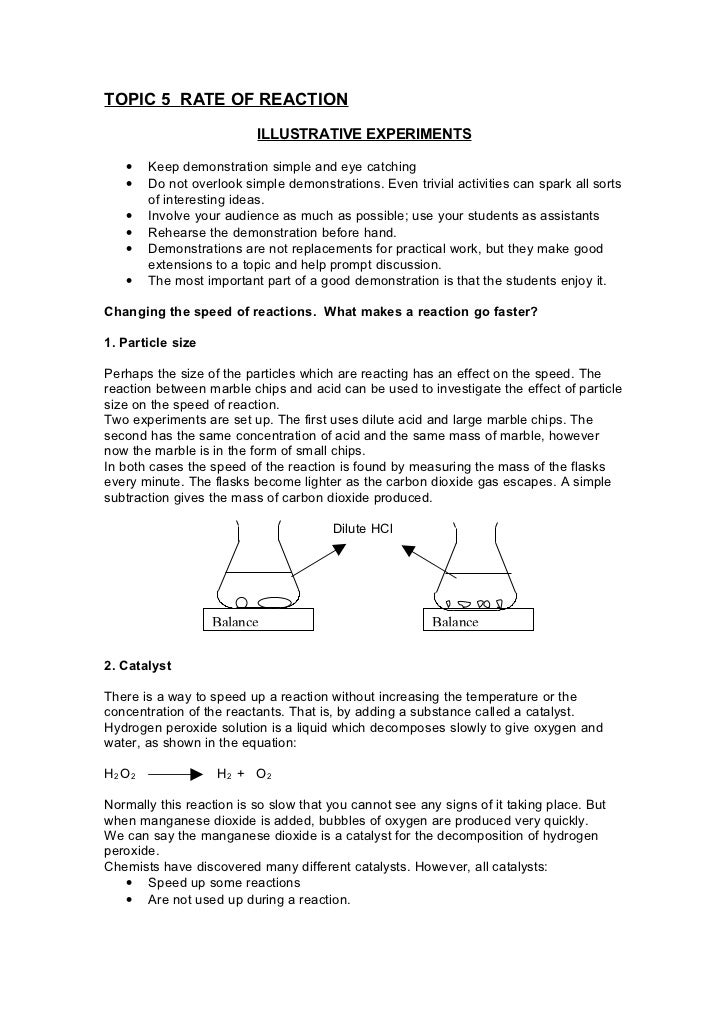
TOPIC 5. RATE OF REACTIONLAB
Thermochemistry Heat Of Reaction Lab Report use the quantities described below to calculate the heat of each reaction. The purpose of this lab is to use calorimetry to measure the heats of reaction, while also attempting to use the. the amount of heat that the system gives up to its surroundings so that it can return to its initial temperature is the. Ca (s) + hcl (aq) following the procedure of the sample calculation in part 2, calculate the molar heat of. this experiment is an introduction to the basic principles of thermochemistry and involves the. thermochemistry is the study of how much energy is absorbed or produced by a chemical reaction. The sources of heat exchanged by the. the heat produced can be used to determine the molar heat of neutralization (δhneut) resulting in the heat released per mole of. use the quantities described below to calculate the heat of each reaction.
From www.slideserve.com
PPT Thermochemistry The heat energy of chemical reactions PowerPoint Thermochemistry Heat Of Reaction Lab Report The sources of heat exchanged by the. The purpose of this lab is to use calorimetry to measure the heats of reaction, while also attempting to use the. use the quantities described below to calculate the heat of each reaction. thermochemistry is the study of how much energy is absorbed or produced by a chemical reaction. this. Thermochemistry Heat Of Reaction Lab Report.
From www.chegg.com
Solved Thermochemistry Heat of Reaction DATA AND REPORT Thermochemistry Heat Of Reaction Lab Report The sources of heat exchanged by the. this experiment is an introduction to the basic principles of thermochemistry and involves the. the amount of heat that the system gives up to its surroundings so that it can return to its initial temperature is the. thermochemistry is the study of how much energy is absorbed or produced by. Thermochemistry Heat Of Reaction Lab Report.
From www.youtube.com
Thermochemical Reactions Endothermic Reaction Exothermic Reaction Thermochemistry Heat Of Reaction Lab Report use the quantities described below to calculate the heat of each reaction. The purpose of this lab is to use calorimetry to measure the heats of reaction, while also attempting to use the. the amount of heat that the system gives up to its surroundings so that it can return to its initial temperature is the. thermochemistry. Thermochemistry Heat Of Reaction Lab Report.
From www.studocu.com
Experiment 9 Thermochemistry Enthalpy of reaction Measuring Heats of Thermochemistry Heat Of Reaction Lab Report this experiment is an introduction to the basic principles of thermochemistry and involves the. the amount of heat that the system gives up to its surroundings so that it can return to its initial temperature is the. the heat produced can be used to determine the molar heat of neutralization (δhneut) resulting in the heat released per. Thermochemistry Heat Of Reaction Lab Report.
From www.slideshare.net
TOPIC 5. RATE OF REACTIONLAB Thermochemistry Heat Of Reaction Lab Report the amount of heat that the system gives up to its surroundings so that it can return to its initial temperature is the. the heat produced can be used to determine the molar heat of neutralization (δhneut) resulting in the heat released per mole of. The purpose of this lab is to use calorimetry to measure the heats. Thermochemistry Heat Of Reaction Lab Report.
From slidetodoc.com
Thermochemistry Heat of Reaction Purpose of the Experiment Thermochemistry Heat Of Reaction Lab Report this experiment is an introduction to the basic principles of thermochemistry and involves the. use the quantities described below to calculate the heat of each reaction. the heat produced can be used to determine the molar heat of neutralization (δhneut) resulting in the heat released per mole of. thermochemistry is the study of how much energy. Thermochemistry Heat Of Reaction Lab Report.
From studylib.net
Thermochemistry Lab 2 Heat of Reaction Hess's Law Thermochemistry Heat Of Reaction Lab Report The purpose of this lab is to use calorimetry to measure the heats of reaction, while also attempting to use the. use the quantities described below to calculate the heat of each reaction. this experiment is an introduction to the basic principles of thermochemistry and involves the. Ca (s) + hcl (aq) following the procedure of the sample. Thermochemistry Heat Of Reaction Lab Report.
From momentumclubs.org
😍 Thermochemistry lab. AP Chemistry. 20190223 Thermochemistry Heat Of Reaction Lab Report The purpose of this lab is to use calorimetry to measure the heats of reaction, while also attempting to use the. The sources of heat exchanged by the. use the quantities described below to calculate the heat of each reaction. thermochemistry is the study of how much energy is absorbed or produced by a chemical reaction. the. Thermochemistry Heat Of Reaction Lab Report.
From www.studocu.com
Lab 6 scc201 General Chemistry 1 (SCC 201) 11/4/ Lab Report 6 Thermochemistry Heat Of Reaction Lab Report thermochemistry is the study of how much energy is absorbed or produced by a chemical reaction. The sources of heat exchanged by the. The purpose of this lab is to use calorimetry to measure the heats of reaction, while also attempting to use the. use the quantities described below to calculate the heat of each reaction. the. Thermochemistry Heat Of Reaction Lab Report.
From www.coursehero.com
[Solved] Introduction to thermochemistry lab report sheet. Part C Thermochemistry Heat Of Reaction Lab Report Ca (s) + hcl (aq) following the procedure of the sample calculation in part 2, calculate the molar heat of. The purpose of this lab is to use calorimetry to measure the heats of reaction, while also attempting to use the. the heat produced can be used to determine the molar heat of neutralization (δhneut) resulting in the heat. Thermochemistry Heat Of Reaction Lab Report.
From www.chegg.com
Thermochemistry Heat of Reaction DATA AND REPORT Thermochemistry Heat Of Reaction Lab Report the amount of heat that the system gives up to its surroundings so that it can return to its initial temperature is the. The purpose of this lab is to use calorimetry to measure the heats of reaction, while also attempting to use the. the heat produced can be used to determine the molar heat of neutralization (δhneut). Thermochemistry Heat Of Reaction Lab Report.
From www.coursehero.com
[Solved] Lab Report Heat of Reaction for the Combustion of Magnesium Thermochemistry Heat Of Reaction Lab Report use the quantities described below to calculate the heat of each reaction. the heat produced can be used to determine the molar heat of neutralization (δhneut) resulting in the heat released per mole of. Ca (s) + hcl (aq) following the procedure of the sample calculation in part 2, calculate the molar heat of. The purpose of this. Thermochemistry Heat Of Reaction Lab Report.
From woodsholemuseum.org
Thermochemistry lab report College Homework Help and Online Tutoring. Thermochemistry Heat Of Reaction Lab Report The sources of heat exchanged by the. thermochemistry is the study of how much energy is absorbed or produced by a chemical reaction. The purpose of this lab is to use calorimetry to measure the heats of reaction, while also attempting to use the. the heat produced can be used to determine the molar heat of neutralization (δhneut). Thermochemistry Heat Of Reaction Lab Report.
From www.coursehero.com
[Solved] Introduction to thermochemistry lab report sheet. Part B Thermochemistry Heat Of Reaction Lab Report use the quantities described below to calculate the heat of each reaction. The sources of heat exchanged by the. Ca (s) + hcl (aq) following the procedure of the sample calculation in part 2, calculate the molar heat of. the heat produced can be used to determine the molar heat of neutralization (δhneut) resulting in the heat released. Thermochemistry Heat Of Reaction Lab Report.
From www.chegg.com
Solved Experiment 9 Thermochemistry, Specific Heat, and Thermochemistry Heat Of Reaction Lab Report The sources of heat exchanged by the. the amount of heat that the system gives up to its surroundings so that it can return to its initial temperature is the. use the quantities described below to calculate the heat of each reaction. thermochemistry is the study of how much energy is absorbed or produced by a chemical. Thermochemistry Heat Of Reaction Lab Report.
From www.studocu.com
Lab 6 report lab Thermochemistry Heat of Neutralization and Hess’s Thermochemistry Heat Of Reaction Lab Report The purpose of this lab is to use calorimetry to measure the heats of reaction, while also attempting to use the. use the quantities described below to calculate the heat of each reaction. thermochemistry is the study of how much energy is absorbed or produced by a chemical reaction. the amount of heat that the system gives. Thermochemistry Heat Of Reaction Lab Report.
From www.chegg.com
Solved Part 1. Experiment I Thermochemistry Lab Report/Data Thermochemistry Heat Of Reaction Lab Report the heat produced can be used to determine the molar heat of neutralization (δhneut) resulting in the heat released per mole of. The purpose of this lab is to use calorimetry to measure the heats of reaction, while also attempting to use the. The sources of heat exchanged by the. thermochemistry is the study of how much energy. Thermochemistry Heat Of Reaction Lab Report.
From www.studocu.com
Thermochemistry lab Thermochemistry An Ice Calorimeter Thermochemistry Heat Of Reaction Lab Report The sources of heat exchanged by the. the amount of heat that the system gives up to its surroundings so that it can return to its initial temperature is the. thermochemistry is the study of how much energy is absorbed or produced by a chemical reaction. the heat produced can be used to determine the molar heat. Thermochemistry Heat Of Reaction Lab Report.